In March 2019, Drug and Device Watch published an article titled “The FDA’s Hidden Database Protects Companies, Reduces Medical Device Recalls”. In this article, we discussed the thousands, possibly millions, of medical device injury and malfunction reports stored in a hidden database. The U.S. Food and Drug Administration (FDA) maintains...
Concerns over Breast Implant Illness Prompt FDA Investigation
The U.S. Food and Drug Administration (FDA) is reacting to reports from a grassroots movement comprised of real life patients who claim they developed breast implant illness. After being dismissed by the medical community, the women turned to Facebook and other social media platforms to spark attention and action. Breast...
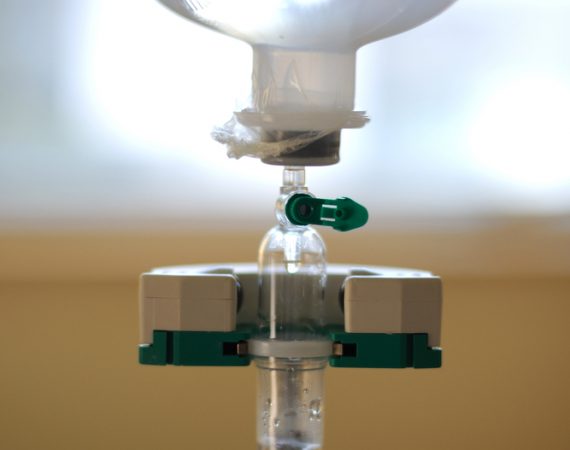
Urgent Device Recall: Chemotherapy Patients May be at Immediate Risk
ICU Medical, Inc. recently issued an urgent device recall for their hazardous closed drug delivery systems used in administering chemotherapy to cancer patients. Chemotherapy patients who receive treatment through a closed delivery system are in danger, and as such, the U.S. Food and Drug Administration (FDA) has classified this recall...
The FDA’s Hidden Database Protects Companies, Reduces Medical Device Recalls
In a bombshell report, Kaiser Health News is shining a light on a little-known practice of the U.S. Food and Drug Administration (FDA) that puts Americans at risk. The FDA’s “alternative summary” program is a private reporting procedure that allows manufacturers to potentially avoid medical device recalls. That is because...
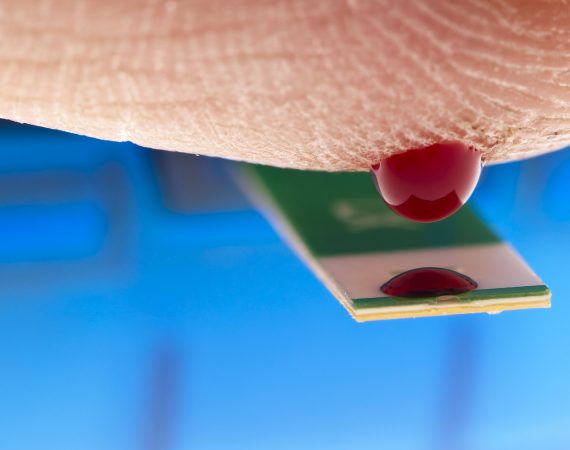
FDA Issues a Recall of Test Strips Sold by Terrific Care/Medex
The U.S. Food & Drug Administration (FDA) reports that medical supply distributor Terrific Care/Medex Supply LLC has issued a voluntary device recall of certain test strips used to monitor patients who take the blood thinner warfarin. This FDA device recall has been classified as a Class I - the most...