Patients with high blood pressure can expect a more severe shortage of the medications they take to manage their condition. Last week, Torrent Pharmaceuticals Ltd. announced an expansion to the already gigantic losartan recall currently in place. Drug and Device Watch details the initial cause of the losartan recall in...
In The News
Defective Infant Product Linked to Numerous Infant Deaths
Fisher-Price recalled their Rock ‘n Play sleeper amid tragic reports of more than two dozen infant deaths associated with the defective infant product. In what many believe to be an attempt at avoiding a recall, the company initially issued a joint statement with the U.S. Consumer Product Safety Commission (CPSC)...
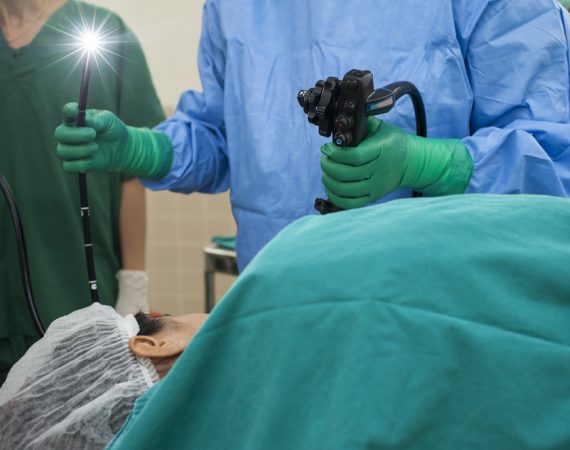
FDA: Medical Scope Infection Rates on the Rise
After years of improvement in the rate of medical scope infections, the U.S. Food and Drug Administration (FDA) recently began receiving reports of superbug infections associated with contaminated medical scopes. In 2015, the high rate of infections prompted the FDA to require medical scope manufacturers to conduct post-market studies on...
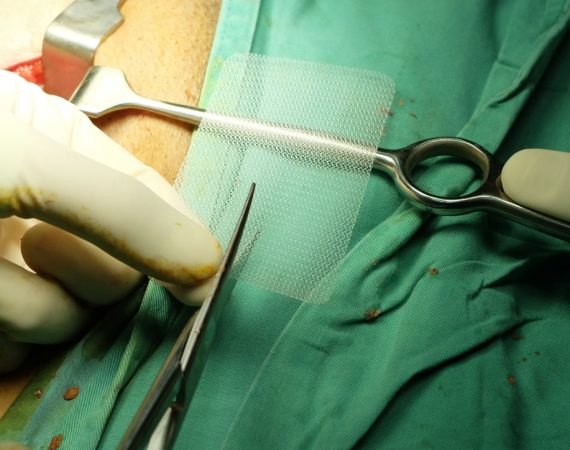
FDA Orders Immediate Stop of Pelvic Surgical Mesh Sales
The latest and final step in an escalating series of warnings and actions about surgical mesh for the treatment of pelvic organ prolapse (POP) came this week as the U.S. Food and Drug Administration (FDA) ordered the manufacturers of some mesh products to stop selling and distributing the devices immediately....
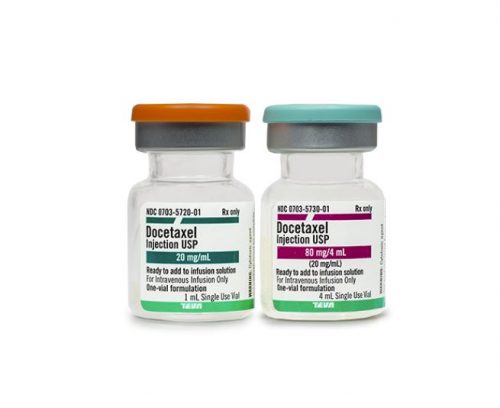
Lawsuits Mount Over Cancer Drug’s FDA Black Box Warning
The manufacturer of a chemotherapy drug called Taxotere is the subject of snowballing litigation after claims that the drug causes permanent alopecia, or hair loss. Though hair loss is a common side effect of cancer treatments, the cancer survivors bringing suit claim the risk of permanency of their hair loss...
FDA Controversial Advice: Take Probable Carcinogens During Blood Pressure Medication Recall
In an unorthodox statement last week, the U.S. Food and Drug Administration (FDA) said it does not object to manufacturers continuing to distribute a slew of drugs involved in a wide-reaching blood pressure medication recall. Their announcement is in response to public concerns of a shortage of blood pressure medicine...
Reports of Device Injury Prompt FDA Chief to Release Hidden Database
In March 2019, Drug and Device Watch published an article titled “The FDA’s Hidden Database Protects Companies, Reduces Medical Device Recalls”. In this article, we discussed the thousands, possibly millions, of medical device injury and malfunction reports stored in a hidden database. The U.S. Food and Drug Administration (FDA) maintains...
Forward Momentum on Bard Hernia Mesh Cases
The federal judge presiding over the consolidated Bard hernia mesh cases has issued a case management order approving 12 cases for the process of discovery, or evidence gathering, to serve as examples of the hundreds of similar cases that will follow. Currently, more than two hundred cases are pending against...
Concerns over Breast Implant Illness Prompt FDA Investigation
The U.S. Food and Drug Administration (FDA) is reacting to reports from a grassroots movement comprised of real life patients who claim they developed breast implant illness. After being dismissed by the medical community, the women turned to Facebook and other social media platforms to spark attention and action. Breast...
Urgent Device Recall: Chemotherapy Patients May be at Immediate Risk
ICU Medical, Inc. recently issued an urgent device recall for their hazardous closed drug delivery systems used in administering chemotherapy to cancer patients. Chemotherapy patients who receive treatment through a closed delivery system are in danger, and as such, the U.S. Food and Drug Administration (FDA) has classified this recall...
The FDA’s Hidden Database Protects Companies, Reduces Medical Device Recalls
In a bombshell report, Kaiser Health News is shining a light on a little-known practice of the U.S. Food and Drug Administration (FDA) that puts Americans at risk. The FDA’s “alternative summary” program is a private reporting procedure that allows manufacturers to potentially avoid medical device recalls. That is because...
Defibrillator Recall: Wearable Defibrillators Recalled after Injuries and Deaths Reported
Zoll Medical Corporation issued a recall of their wearable defibrillator LifeVest 4000 after the U.S. Food & Drug Administration (FDA) received reports of two deaths and an undisclosed number of miscellaneous injuries. This defibrillator recall is intended to prevent a potentially deadly malfunction. What is the LifeVest 4000? The Zoll...