Over the past several months, a number of Metformin recalls have caused concern and near-panic in the healthcare community and among consumers. Various batches of Metformin have been recalled due to containing higher-than-acceptable levels of the carcinogen N-Nitrosodimethylamine (NDMA). Now, the already significant diabetes drug recall has expanded once again....
Another Thyroid Medication Recall Due to Sub Potency
The U.S. Food and Drug Administration (FDA) has announced another thyroid medication recall due to the drugs being sub potent. This FDA recall includes two lots of medication manufactured by Acella Pharmaceuticals, LLC. The Acella recall follows just weeks after RLC Labs, Inc. announced a hypothyroidism medication recall of certain...
Another Metformin Recall Due to Carcinogen Concerns
The U.S. Food and Drug Administration (FDA) has identified yet another metformin recall due to higher-than-acceptable levels of carcinogen N-Nitrosodimethylamine (NDMA). This marks the sixth drugmaker to recall metformin and pull lots from the market. Here is our continuing coverage of this medication recall. Another Metformin Recall Due to Carcinogen...
Sundial Herbal Products Recalled as ‘Unapproved Drugs’
If you regularly purchase herbs and supplements as part of your healthcare regimen, take note of this massive recall! Sundial Herbal Products is recalling all of their products with a manufacture date of 2014 or later. The recall follows the U.S. Food and Drug Administration (FDA) deeming them “unapproved drugs.”...
FDA Announces More Metformin Recalls Due to Carcinogen
The U.S. Food and Drug Administration (FDA) has announced more Metformin recalls due to potential contamination with the carcinogen N-Nitrosodimethylamine (NDMA). The latest recall is the fifth involving extended-release Metformin tablets. For consumers who rely on Metformin to manage their Type 2 diabetes, Metformin recalls are reasonably concerning. Here is...
Thyroid Medication Recall: ‘Superpotent’ Hypothyroidism Drug Causes Hyperthyroidism
Acella Pharmaceuticals, LLC is voluntarily recalling certain lots of the hypothyroid medication NP Thyroid. According to the company announcement, the thyroid medication recall follows tests showing certain lots are “superpotent.” The affected products may have up to 115.0% more liothyronine (T3) than what is on the label. Read on to...
Another Heartburn Medication Recall Due to NDMA Concerns
Following on the heels of massive Zantac and other heartburn medication recalls, yet another medication is being recalled due to concerns about NDMA (N-Nitrosodimethylamine). The U.S. Food and Drug Administration (FDA) announced a recall of certain lots of Amneal Pharmaceuticals, LLC Nizatidine Oral Solution. Here is what we know about...
Zantac Recall Update: FDA Calls for Removal of All Products from the Market
After months of investigating ranitidine products for the presence of a known human carcinogen, the U.S. Food and Drug Administration (FDA) is now calling for complete removal of all products containing ranitidine from the market. In January 2020, we wrote about the Zantac recall and worries about contamination with N-Nitrosodimethylamine...
FDA Urged to Recall Metformin after Third Party Testing Shows NDMA in 16 Batches
Pharmaceutical companies are under more pressure than ever due to concerns about contamination and the presence of unsafe ingredients. Readers will no doubt recall notable recent medication recalls like Zantac (ranitidine), Valsartan and Belviq. Now, another popular drug, Metformin, is joining those ranks after third party tests found the probable...
Weight Loss Drug Belviq Recall: Drug Linked to ‘Increased Occurrence of Cancer’
Popular weight loss drug Belviq is being recalled after a safety clinical trial showed an “increased occurrence of cancer.” Following the trial, the U.S. Food and Drug Administration (FDA) requested a Belviq recall from Eisai Co., the manufacturer. Eisai acceded to the FDA’s request, though the company claims that their...
More Antacid Recalls Issued due to Trace Elements of NDMA
On the heels of notable recent medication recalls like Zantac, the U.S. Food and Drug Administration (FDA) is recalling even more medications due to possible trace elements of NDMA. NDMA (Nitrosodimethylamine) is a probable human carcinogen, meaning it may cause cancer. The presence of NDMA in Zantac has made waves...
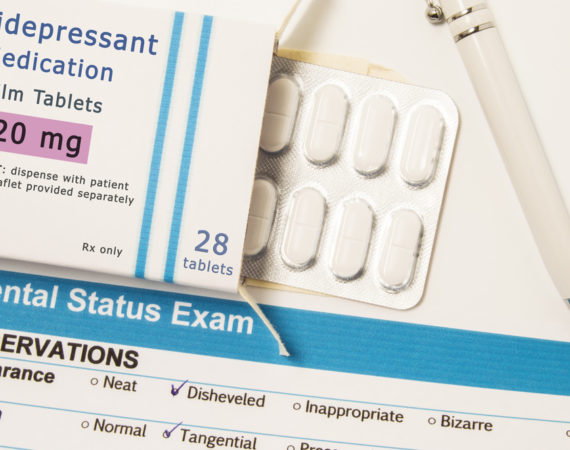
FDA Recall Alert: Antidepressant Mirtazapine Affected by Dangerous Labeling Error
The U.S. Food and Drug Administration (FDA) has announced that Aurobindo Pharma USA, Inc. is voluntarily recalling a single lot of antidepressant medication, Mirtazapine. According to the FDA notice, the recall is due to a dangerous labeling error. Patients could take a higher dose of the antidepressant than what is...