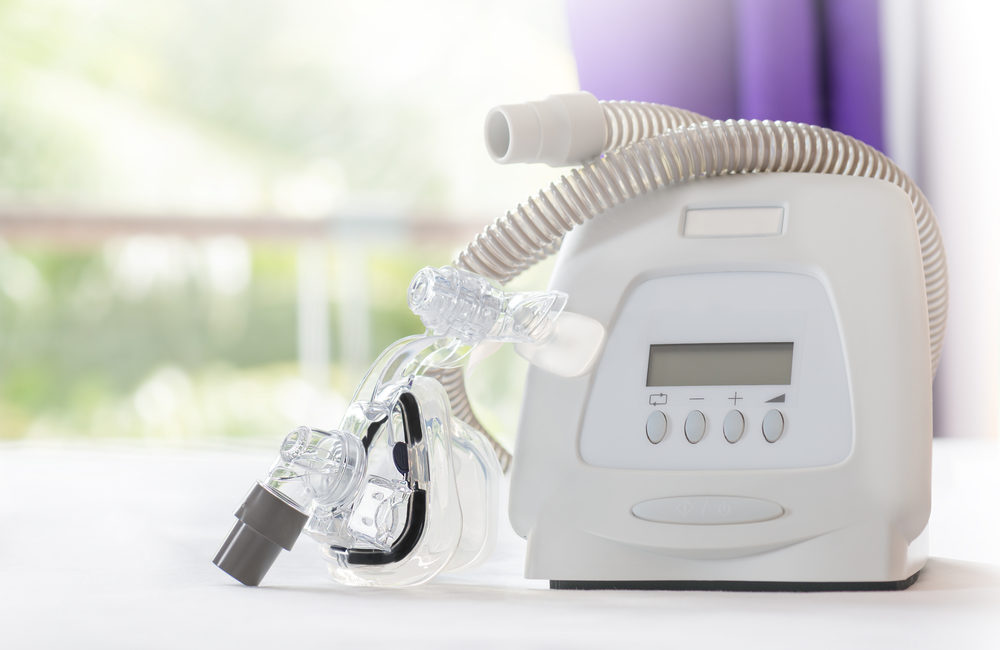
Popular manufacturer, Philips Respironics, is recalling millions of CPAP (continuous positive airway pressure) and BiPAP (Bilevel Positive Airway Pressure) machines. The machines apparently contain foam that could be dangerous or could even cause cancer. Read on to learn more about the CPAP recall and how it could affect you if you use a CPAP or BiPAP machine.
CPAP Recall Information
The Philips CPAP recall primarily affects DreamStation machines. These CPAP and BiPAP machines have a type of foam inside them that dampens the sound and reduces vibration. The foam is a polyester-based polyurethane (PE-PUR).
Swallowing or inhaling the foam can cause headaches and breathing problems. The foam itself may also be carcinogenic, meaning it could increase the risk of the patient developing certain types of cancer. There is also concern that the gases released by the foam may be harmful.
The U.S. Food and Drug Administration (FDA) has received patient complaints stating that the foam can break down and the patient may swallow or inhale it. Patients have reported black particles or debris in the tubing. The breakdown of the foam may occur during cleaning or due to high levels of humidity building up in the machine.
The FDA has received patient complaints of:
- Headache
- Cough
- Chest pressure
- Upper airway irritation
- Sinus infection
Currently, there is no positive correlation between these symptoms and the CPAP machines. However, the FDA notes that these and other symptoms may be related to particulate exposure. Particulate exposure can cause:
- Irritation (skin, eyes, respiratory tract)
- Inflammatory response
- Headache
- Asthma
- Carcinogenic effects to organs
In addition, the FDA warns that exposure to potentially toxic chemicals in the device’s air pathway (tubing) can also cause symptoms including:
- Headache
- Dizziness
- Irritation (eyes, nose, skin, respiratory tract)
- Hypersensitivity
- Nausea and vomiting
- Toxic or carcinogenic effects
- Devices Included in the Recall
So far, the FDA has no reports of fatal injuries. However, they are warning patients out of an abundance of caution, to talk to their healthcare providers if they use one of the recalled machines and experience any of these symptoms.
Machines Included in the Recall
The machines included in this recall are Philips, and were manufactured between 2009 and 2021. The devices and models are as follows:
- Continuous Ventilator, Minimum Ventilatory Support (facility use) – E30
- Continuous Ventilator, Non-Life Supporting:
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C-Series ASV
- C-Series S/T and AVAPS
- OmniLab Advanced+
- Noncontinuous Ventilator
- SystemOne (Q-Series)
- DreamStation
- DreamStation Go
- Dorma 400
- Dorma 500
- REMstar SE Auto
- Continuous Ventilator
- Trilogy 100
- Trilogy 200
- Garbin Plus, Aeris, LifeVent
- Continuous Ventilator Minimum Ventilatory Support (facility use) – A-Series BiPAP V30 Auto
- Continuous Ventilator, Non-Life Supporting
- A-Series BiPAP A40
- A-Series BiPAP A30
Continuing Investigation into CPAP Machines
The FDA is continuing to investigate the scope of the CPAP recall. They are analyzing medical reports from 2009-2021, are evaluating mitigation strategies, and are monitoring supply and use. About half of the 3-4 million machines included in the CPAP recall are used in the United States. Of the devices in the recall, 80% are used by patients with sleep apnea. The other 20% are life-supporting ventilators.
Anyone using one of the recalled CPAP or BiPAP machines should talk to their doctor about possible alternatives. You should never stop using a CPAP, BiPAP, or ventilator without talking to your doctor first.
If you clean your CPAP machine or accessories, make sure you follow the manufacturer’s guidelines. Avoid Ozone cleaners, which can cause the foam to breakdown faster. If you notice any particles or signs of breakdown, report the issues to the FDA via their MedWatch Voluntary Reporting Form.
At Drug and Device Watch, we are continuing to monitor the status of this CPAP recall. We will continue to update our readers as new information becomes available. As always, if you have questions about a defective medical device and your legal rights, you can reach out to us by completing our online contact form.