ICU Medical, Inc. recently issued an urgent device recall for their hazardous closed drug delivery systems used in administering chemotherapy to cancer patients. Chemotherapy patients who receive treatment through a closed delivery system are in danger, and as such, the U.S. Food and Drug Administration (FDA) has classified this recall as a Class I – the most serious recall that can be issued – due to credible and urgent risk to life.
What is the Urgency?
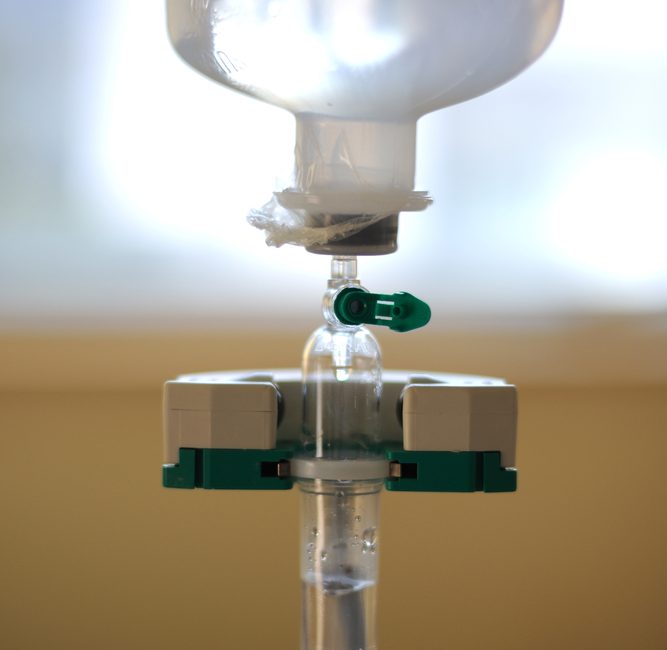
ICU Medical initially voluntarily recalled certain ChemoLock and ChemoClave Vial Spikes after it was determined that use of these delivery systems may result in “burr particulate”, that is tiny particles of plastic, entering the bloodstream through the IV line. The burr particulate can cause serious complications, such as a life-threatening embolism. Any chemotherapy patient who has received treatment with ICU Medical devices may be at-risk for the plastic particles.
Microplastics found in our oceans and food sources are increasingly making headlines. Experts continue to debate their impact on the human body, however, the presence of plastic particles delivered directly into the blood is universally accepted as a life-threatening, dangerous situation. Due to this risk, the FDA and ICU Medical have agreed on the urgent device recall to reduce the risk to patients.
Which Devices Are Being Recalled?
Closed drug delivery systems are not available for home use but are used in at least 41 percent of cancer treatment facilities across the country. Any cancer patient who receives drugs intravenously may have been treated with these defective devices without their knowledge.
ICU Medical distributed the devices to cancer treatment facilities from August 2018 to February 2019. The individual devices are described as follows:
- Oncology Kit with 12” Ext Set w/Spiros w/ red cap, clamp, graduated adapter, Chemoclave vented vial spike 20mm, ChemoClave vented vial spike 13 mm, Spiros w/red cap, Item no. CH 3943
- Oncology Kit with 12” Ext Set w/Spiros with red cap, clamp, grandated adapter, Chemoclave vented vial spike 20mm, ChemoClave vented vial spike 13 mm, Item no 3944
- ChemoClave vented vial spike, 20 mm, Item no CH-70S
- ChemoClave vial spike, 20 mm, Item no CH-80S
- ChemoClave vial spike, 20 mm, Item no CL-80S
- ChemoClave vial spike, 20 mm 10 units, Item no CL-80S-10
- ChemoClave closed vial spike w/skirt, 4 units, Item no CL-80S-4
- ChemoClave closed vial spike w/skirt, 5 units, Item no CL-80S-5
- ChemoClave closed vial spike w/skirt , 20mm, Item no Z7148
Healthcare providers and cancer treatment facilities are urged to check their closed delivery systems to ensure that they are not using a product included in this urgent device recall.
Who May be in Danger?
Chemotherapy patients are most likely to be in danger. Specifically those who:
- Receive treatment that is so toxic as to require the use of a closed delivery system.
- Receive chemo using products that may be ChemoClave brand.
Medical professionals use closed drug delivery systems in general for their own safety. The drugs in chemotherapy cocktails are effective because they are hazardous to both cancer and human cells. The device that is being so urgently recalled exists to protect doctors, nurses, and other medical professionals from daily exposure to the substances cancer patients endure.
Medical professionals are not endangered by this product, as the defect lies with the substances inside the closed vial.
The faulty devices were distributed to cancer treatment facilities nationwide. Any chemotherapy patient who receives their treatment through a closed drug delivery system is at risk and should have his or her blood tested for the presence of burr particulate as soon as possible. ICU Medical distributed more than two thousand of the defective drug delivery vials nationwide.
Dangers of Plastic Particles in the Bloodstream
Embolism, or a blood clot, is the main concern when plastic enters the bloodstream directly through an IV line. The plastic particles can clump together and wedge in a blood vessel causing a pulmonary embolism or stroke. Basically, blocked blood vessels that rob any vital organ of blood can mean death if the blockage is not treated immediately.
The introduction of foreign material into the blood means the patient doesn’t need to have any risk factors for clotting in order for clotting to be a concern. The plastic particles are just as dangerous to a patient with no risk of blood clots as they are to a high-risk patient. The no-risk patient may not recognize symptoms in time to seek life-saving medical attention.
The two deadliest blood clot conditions are pulmonary embolism and stroke. Recognize the signs of a pulmonary embolism by observing for:
- Sudden appearance of shortness of breath that gets worse with exertion
- Intense chest pain that doesn’t go away with rest
- Productive, bloody cough
Recognize the signs of a stroke by looking for:
- Incoherent speech
- Sudden difficulty understanding speech
- Paralysis or numbness of the face, arm, or leg often on just one side
- Trouble seeing in one or both eyes
- Sudden, severe headache which may be accompanied with vomiting
- Trouble walking or sudden loss of coordination
- Sudden dizziness
If you or someone you love is being treated with chemotherapy and you notice any of these warning signs, get medical attention right away.
Additional Health Concerns of Plastic Particles in the Blood
Plastic particles small enough to be carried through the bloodstream will be small enough to transfer to, and perhaps collect in, any organ of the body. If the particles don’t clot together but flow freely throughout the body, the most likely place they could collect is in the liver. The concern at that point is that the content of the plastic itself could leach chemical contaminants throughout the body.
The chemicals in plastics may suppress the immune system, which is of particular concern to chemotherapy patients who already have suppressed immune systems. Even if accumulated plastics don’t result in a life-threatening situation in the short-term, long-term exposure to plastic via the bloodstream may still result in a dangerous situation for cancer patients.
What to Do About This Urgent Device Recall?
Medical professionals have been urged discontinue use of recalled products and to return the devices to the manufacturer. Removing the defective devices from use should reduce the risk associated with the device, which was chemotherapy treatments administered between August 2018 and February 2019.
To be safe, if you or a loved one is a chemotherapy patient, ask your medical provider to disclose the brand of the drug delivery vial. Don’t hesitate to tell them about the FDA recall. An urgent device recall like this can be deadly for patients already fighting for their lives, so speak up! The stakes are too high to assume that your healthcare provider is aware of the recall, or has discontinued use of the product.
Consult with a Medical Device Recall Lawyer
At this time, there have been no adverse events reported to the FDA associated with these faulty drug vials. However, this urgent device recall is ongoing. If you or someone you love is battling cancer with chemotherapy treatment, and blood tests have been positive for plastic particles, it is important to contact a drug and device attorney to learn more.
You have rights as a patient, including the right to be treated with devices that are safe, effective, and not recalled. Find out more about this urgent device recall and your legal rights by contacting Drug and Device Watch. You may be entitled to compensation if you have been harmed by plastic particles caused by defective medical devices. Contact us online, or call us at 1-888-458-6825 for a free consultation.
Sources:
- https://www.prnewswire.com/news-releases/icu-medical-issues-a-voluntary-nationwide-recall-of-certain-lots-of-chemolock-and-chemoclave-vial-spikes-due-to-the-potential-for-burr-particulate-300800605.html
- https://www.cancertherapyadvisor.com/home/cancer-topics/general-oncology/closed-system-transfer-devices-cstds-for-chemotherapy-more-than-an-added-layer-of-protection/2/
- https://www.mayoclinic.org/diseases-conditions/pulmonary-embolism/symptoms-causes/syc-20354647
- https://www.mayoclinic.org/diseases-conditions/stroke/symptoms-causes/syc-20350113
- https://news.sky.com/story/humans-swallowing-tiny-plastic-particles-in-their-food-study-finds-11532893