The U.S. Food and Drug Administration (FDA) is categorizing Medtronic’s septostomy catheter recall as Class I. A Class I medical device recall is the most serious, meaning that use of the medical device could cause serious injuries or death. Here is what we know about the recall.
Medical Device Recall for Medtronic Septostomy Catheters
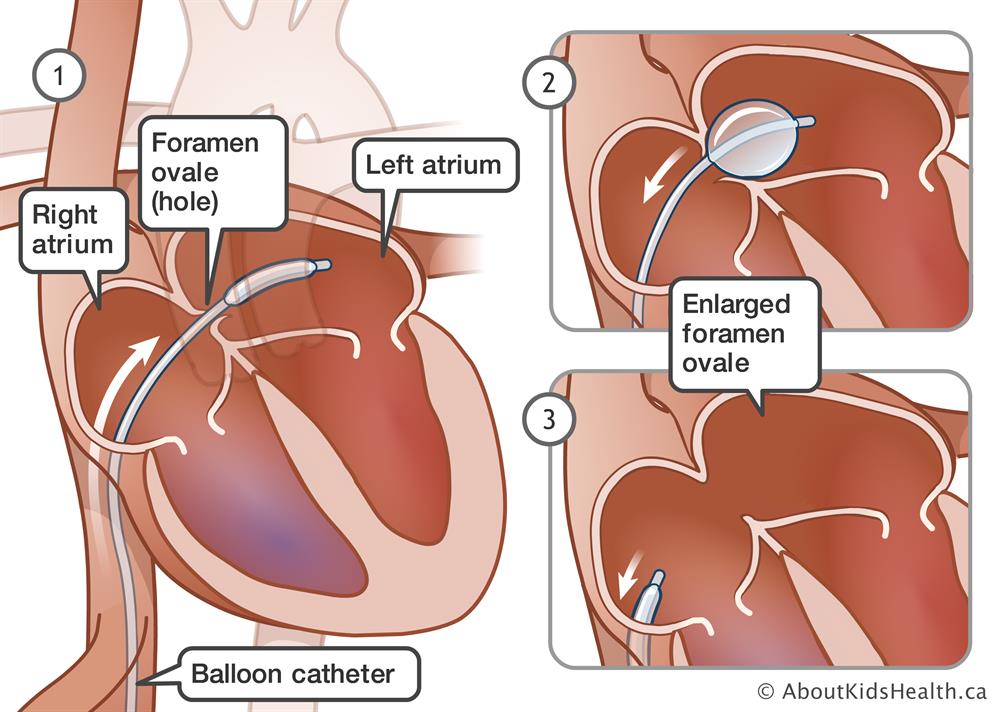
In August 2020, Medtronic initiated a medical device recall for Rashkind Balloon Septostomy Catheters. These catheters are used to create or enlarge an atrial septal defect in patients suffering from Cyanotic Congenital heart defects.
Medtronic is recalling the septostomy catheters because of quality issues. The FDA recall notice says that these issues could cause the device to break, separate or fail during use. Any of these problems could have serious adverse health consequences for the patient, such as damage to the blood vessels or death.
So far, the FDA reports at least two injuries and one death associated with the Rashkind Balloon Septostomy Catheters. Medtronic has halted manufacturing and distribution of the catheters due to factors not related to this recall.
Rashkind Balloon Septostomy Catheter Recall Information
Healthcare providers and patients can identify the catheters subject to this Class I recall by the following:
Rashkind Balloon Septostomy Catheter 6F
- Product Number 008764
Rashkind Balloon Septostomy Catheter 5F
- Product Number 007160
Rashkind Balloon Septostomy Catheter 4F
- Product Number 007161
There are various lot numbers associated with this medical device recall. In total, there are 142 devices included.
The FDA warns that this medical device recall may affect healthcare providers who use Rashkind Balloon Septostomy Catheters, as well as patients undergoing procedures where the device may be used. Medtronic is requesting that affected customers do the following:
- Identify and quarantine all catheters affected by this recall.
- Return all unused products affected by the recall to Medtronic.
- Complete a Customer Confirmation Form and return it to Medtronic.
- Forward the recall notice to all relevant parties.
Patients Who May be at Risk in this Medical Device Recall
As noted above, patients who may be at risk due to this Class I recall include patients who undergo procedures to treat congenital heart defects. These defects are often noted in infants, and treatment may include use of a septostomy catheter procedure. The Rashkind Balloon Septostomy Catheters are designed for pediatric use.
The FDA urges healthcare providers and patients who experience adverse events or quality problems to report their experience through MedWatch: The FDA Safety Information and Adverse Event Reporting Program. Consumers can file a report online, through mail or by fax.
Contact a Medical Device Attorney
Consumers who experience adverse events from the Rashkind Balloon Septostomy Catheters may find it helpful to contact a medical device attorney to discuss their legal rights. Consumers who suffer injuries or death due to a defective medical device may have an actionable legal claim against the manufacturer.
Drug and Device Watch can help you explore your rights and determine if your case is actionable. Find out more by calling us at 1-888-458-6825. You can also reach out to us by completing our online contact form.
Sources:
- https://www.fda.gov/medical-devices/medical-device-recalls/medtronic-recalls-rashkind-balloon-septostomy-catheters-quality-issues
- https://medlineplus.gov/ency/article/001104.htm
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=183535
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=183685
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=183686
- https://www.aboutkidshealth.ca/Article?contentid=1668&language=English#
- https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program