In February 2020, Boston Scientific announced a recall of Imager II 5F Angiographic Catheters. The medical device recall came after the company reported receiving more complaints than usual about the device’s tip becoming detached. Now, the U.S. Food and Drug Administration (FDA) is upgrading the recall to a Class I, which is the most serious classification of recall. A Class I recall means that,
Using the device may cause serious injuries or death.
According to the FDA, there are currently nine reports of injury due to the devices.
Medical Device Recall Upgraded by FDA
The Imager II catheters are used to deliver contrast to major arteries and blood vessels. Essentially, they provide a pathway for contrast to enter the applicable blood vessel or artery.
Boston Scientific designed these particular catheters with a soft tip that reduces the risk of trauma.
Unfortunately, there appears to be a defect resulting in tip detachment during procedures. The detached tips can obstruct blood flow (embolism), cause strokes or be fatal. Timely removal of a detached tip is important. Removal requires additional surgery and often, a more lengthy hospital stay.
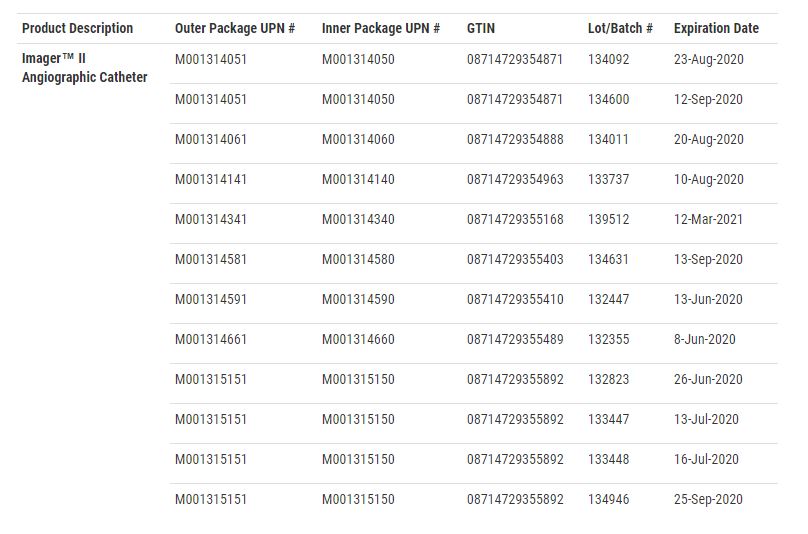
According to the FDA recall notice, the manufacture dates range from July 2018 and November 2019. The recall includes more than 6,100 units. The individual lot codes are as follows:
This is the first Class I recall involving the Imager II catheters. There have been other recalls of Boston Scientific catheters in recent years, but those primarily relate to packaging errors and other less-dangerous faults.
Boston Scientific continues to investigate the cause of tips detaching among the recalled medical devices. They note that the products all meet manufacturing and design standards, and there may be an external reason why the tips are becoming brittle and detaching.
Who is Affected by the Catheter Recall?
Healthcare providers and patients may be affected by the catheter recall. The FDA notes:
- Healthcare providers who use the Imager II catheters use the catheter.
- Patients who have cardiac surgery may experience adverse events during use.
Patients who have cardiac surgery may not be aware of the medical devices surgeons use during surgery. Therefore, patients who have recently had cardiac surgery may want to contact their healthcare provider to find out if they are at risk.
What to Do about the Catheter Recall
In February 2020, Boston Scientific sent a letter to customers alerting them to the recall. The letter included information about the product and lot numbers subject to the recall. The company letter also offered the following instructions:
- Remove any products subject to the recall from hospital inventories
- Stop using products subject to the recall immediately
- Complete a Verification Form (provided) including the quantity of units subject to the recall
- Return all recalled products to Boston Scientific
For patients, tip detachment will likely be noticeable at the time of the cardiac procedure. Even so, doctors may not report the incident to the FDA. Therefore, patients are urged to file adverse events reports through MedWatch: The FDA Safety Information and Adverse Event Reporting Program.
Are You Suffering Due to a Defective Medical Device?
If you have been injured by a defective medical device, it is important to know that you have legal rights. Product manufacturers have a responsibility to make sure their products are safe. When they are defective and cause injuries, the victims can – and should – hold the manufacturer liable.
At Drug and Device Watch, we help injured victims understand their legal rights and options. If you are suffering an injury or illness due to a defective medical device, you may have grounds to file a personal injury claim. Such a claim could provide you with valuable compensation for your injuries and related losses.
To find out if you have an actionable claim, contact Drug and Device Watch to request a free consultation with one of our attorneys. Call us today at 1-888-458-6825, or complete our online form to get started.
Sources: