Medical device manufacturer, Roche Diagnostics, has received emergency use approval (EUA) from the U.S. Food and Drug Administration (FDA) for their Cobas SARS-CoV-2 coronavirus test. The test detects the virus COVID-19 through nasopharyngeal or oropharyngeal swab samples. The test can be used in patients who meet clinical or epidemiological testing criteria for COVID-19.
Roche Coronavirus Test Receives Emergency Use Authorization
With the FDA’s EUA, the coronavirus test can be sent to hospitals and laboratories that use the Cobas 6800 and 8800 automated systems. The purpose of the EUA is to address the urgent medical needs that many communities are facing as the coronavirus continues to spread.
The CEO of Roche, Thomas Schinecker, says,
“Providing quality, high-volume testing capabilities will allow us to respond effectively to what the World Health Organization has characterised as a pandemic. It is important to quickly and reliably detect whether a patient is infected with SARS-CoV-2.”
Not only is this coronavirus test said to be reliable, but it produces results much quicker than initial tests. The Cobas 6800 and 8800 systems can produce results in as little as three hours. During testing, the Cobas 6800 produced 384 results within eight hours. The Cobas 8800 produced 960 results in eight hours.
While the SARS-CoV-2 test has received EUA, it has not yet received full FDA clearance and approval. Roche is also working on a CE-IVD test in areas that accept CE mark approval. This would be for patients who have coronavirus as well as patients who live in areas with a large number of cases.
Current Status of Coronavirus in the United States
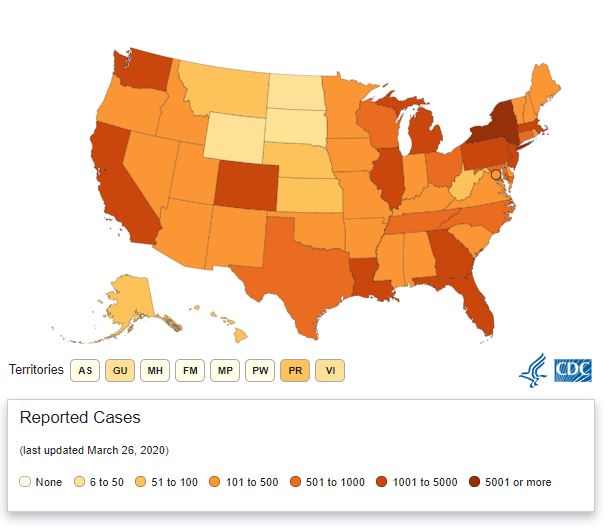
There is no topic hitting the news and social media right now more than coronavirus. The virus has quickly spread from one side of the world to the other. Here in the U.S., the virus is certainly wreaking havoc on communities from coast to coast. A CDC overview of the states that are most affected shows:
In total, there are more than 75,000 cases of coronavirus across all 50 states and U.S. territories. More than 1,000 people have died, and thousands more are in the hospital. The U.S. now has the third-highest rate of coronavirus cases behind only Italy and China.
Coronavirus Tests in the U.S.
According to the CDC, there is an increasing number of laboratories processing coronavirus tests. There are currently 92 public health laboratories (PHLs) in the U.S. that are verified and are offering coronavirus tests. There are laboratories in all 50 states, as well as the District of Columbia, Puerto Rico and Guam. The U.S. Virgin Islands is working on securing a laboratory.
There are also a number of drug and medical device manufacturers who are working on vaccines and treatment options for coronavirus. Any such vaccines or treatments are in development. There is still no FDA-approved vaccine or treatment for coronavirus.
If you are in need of a coronavirus test or have questions, the CDC urges you to contact your local Health Department. They have organized a resource that lists State and Territorial Health Department Websites. Contact your state or territorial Health Department to find out information specific to your area.
Sources:
- https://www.medicaldevice-network.com/news/roche-coronavirus-test-fda-authorisation/
- https://abcnews.go.com/Health/coronavirus-live-updates-diagnosed-covid-19-cases-approach/story?id=69806200&cid=clicksource_4380645_3_takeover_2_bsq_image
- https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- https://www.cdc.gov/publichealthgateway/healthdirectories/healthdepartments.html