The number of coronavirus (COVID-19) cases in the United States continues to rise. As more communities suffer infection, quarantines and general panic, researchers continue to learn whatever they can about how the virus spreads, who is most at risk and how the virus could be prevented. Drug manufacturers are among those most anxious to develop a preventative vaccine or treatment. Of course, with drug safety being such a significant concern, the process could take some time.
Several drug manufacturers are actively working on possible vaccines or treatments to combat the infection. Scientists across the U.S. are working on vaccines, and some promise that they are close to finding a suitable prevention or treatment.
Drug Manufacturers Working on Coronavirus Vaccines or Treatment
Drug manufacturers large and small are moving forward with development and testing of vaccines and treatment options for coronavirus. Two organizations are providing funding to nine pharmaceutical companies – the Biomedical Advanced Research and Development Authority (BARDA) and the National Institute of Allergy and Infectious Diseases (NIAID). Both of these organizations are divisions of federal offices. In addition to funding from major organizations, some companies are funding trials on their own, or are partnering with other companies.
There are currently at least nine drug manufacturers currently developing or testing vaccines. Information about those companies and their progress follows.
Gilead Sciences Inc.
Gilead Sciences Inc. is currently in phase 3 clinical trials of a treatment for coronavirus. The treatment is a drug called remdesivir, which treats symptoms of pneumonia in patients who have coronavirus. In February 2020, the Chinese Food and Drug Administration approved trials. Shortly thereafter, the NIAID also began enrolling patients in a phase 3 randomized, double-blind, placebo-controlled trial. Additional trials in the U.S. are already scheduled for March through May 2020.
GlaxoSmithKline
GlaxoSmithKline (GSK) is a well known drug and medical device manufacturer. They are responsible for one of the most popular flu vaccines, as well as the human papillomavirus (HPV) vaccine. Now, GSK has given the University of Queensland access to their adjuvant platform technology, which can strengthen response to a vaccine. Other pharmaceutical companies are also using the technology to develop possible vaccines.
Inovio Pharmaceuticals Inc.
Inovio Pharmaceuticals Inc. is developing a DNA-based vaccine that is currently in the pre-clinical stages. INO-4800 – the vaccine – could be ready for clinical trials as early as April 2020. There are 3,000 doses currently ready for testing, and the company plans to test in the U.S., China and South Korea. Inovio says that they may have initial results as early as fall, with around one million doses available by the end of the year.
Johnson & Johnson
Johnson & Johnson is working on an unnamed vaccine. J&J is working with BARDA to test the possible vaccine, which could start clinical trials by the end of 2020. J&J says that they are working with partners around the world so that if the vaccine is approved, it will be accessible to China and other parts of the world affected by coronavirus.
Moderna Inc.
Moderna Inc. is working on an RNA-based vaccine with the help of funding from the Coalition for Epidemic Preparedness (CEPI). In February 2020, Moderna sent the first batch of the vaccine, called mRNA-1273 to the NIAID to begin phase 1 testing. In March 2020, Moderna hopes to begin clinical trials, which will follow patients for one year after receiving the vaccine. The trial will be done at Kaiser Permanente Washington Health Research Institute in Seattle, Washington.
Regeneron Pharmaceuticals Inc.
In February 2020, Regeneron announced that they were working on a possible treatment for coronavirus. The company is using monoclonal antibodies to develop vaccine. Regeneron uses a VelocImmune platform, which conducts preclinical testing on genetically-engineered mice. The mice have “humanized” immune systems. Regeneron hopes to have hundreds of thousands of doses ready for human tests by August 2020.
Sanofi
Sanofi is working with BARDA to develop a vaccine for coronavirus. The vaccine is currently in the preclinical stages. The vaccine would prevent severe acute respiratory syndrome (SARS) caused by coronavirus COVID-19. Sanofi hopes to have the vaccine in phase 1 clinical trials by March 2021.
Takeda Pharmaceutical Company Ltd.
Takeda has recently announced that they are working on a possible treatment for coronavirus COVID-19. The company plans to test TAK-888, a treatment using hyperimmune globulins, on patients who have recovered from coronavirus. The testing, which will take place in Georgia, will require access to plasma from recovering patients.
Coronavirus Continues to Spread in U.S.
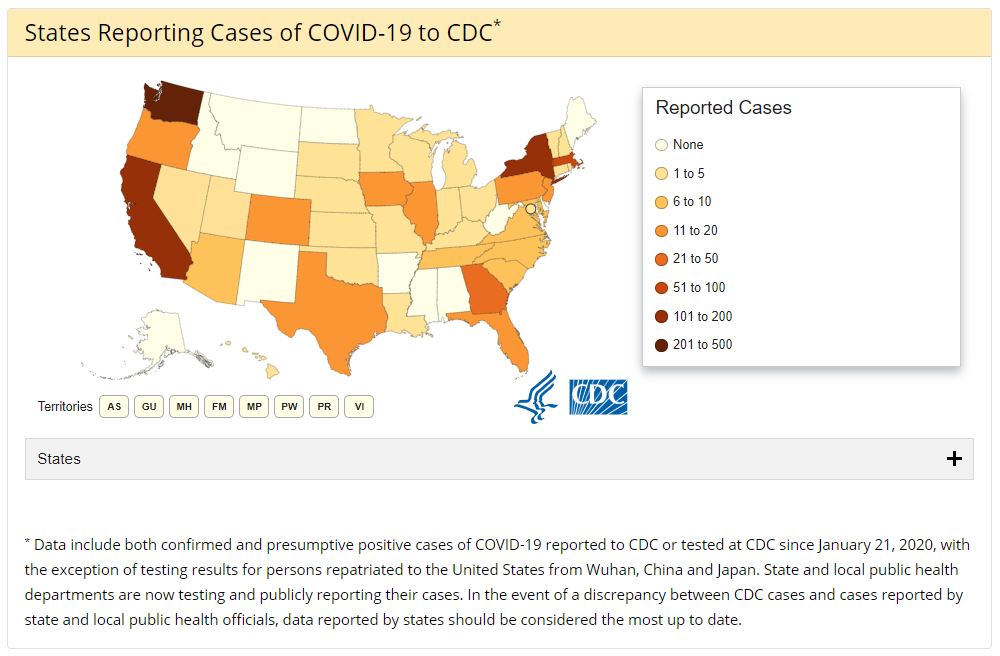
While drug manufacturers and scientists continue to work toward understanding COVID-19, the American public has little choice but to wait. According to the CDC, there are close to 1,000 cases of coronavirus in the U.S. The virus has caused at least 29 deaths.
Officials in many states are monitoring numerous other potential cases, which means the number could be much higher. Across the world, there are more than 126,000 cases spread across 122 countries and territories. There have been more than 4,600 deaths worldwide.
What You Can do to Prevent Coronavirus
In the absence of an FDA approved vaccine or treatment, the best thing that you can do is be aware and prepared. Make sure that you and your loved ones are aware of the symptoms of coronavirus. The most common symptoms are cough, fever and shortness of breath.
If someone in your household is sick, make sure that they stay away from others. If someone in your community is sick, they may need to be under quarantine until they are well. Separating people who are sick from those who are not is one of the best ways to prevent spreading of coronavirus.
Additionally, make sure that your household uses good hand hygiene. Wash hands thoroughly with soap and water, especially after using the bathroom, coughing or sneezing. In between washes, use hand sanitizer with at least 60 percent alcohol. If you are sick, do not prepare food or clean up after others who are not sick.
Also, be aware that certain populations are more at risk for developing coronavirus than others. Anyone with chronic health conditions may be at a higher risk. This includes people with asthma, lung disease, heart disease or diabetes. Elderly individuals – especially those in nursing homes – are also at a higher risk of developing a more serious form of coronavirus. While research is still limited, pregnant women and young children should certainly be monitored closely if they develop symptoms of coronavirus.
Sources:
- https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06
- https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html
- https://www.nursinghomeabusecenter.org/news/coronavirus-outbreak-nursing-home-need-precautions/
- https://www.birthinjuryguide.org/2020/03/women-concerned-birth-coronavirus-outbreak/