Mavidon – a large manufacturer of medical devices – is recalling all of their products worldwide due to possible contamination with Burkholderia cepacia. This worldwide medical device recall is making waves in the healthcare industry. Here is what we currently know about the recall.
Worldwide Recall of Mavidon Medical Devices
Mavidon is voluntarily recalling all lots of all products immediately due to possible contamination with a dangerous pathogen. The recall includes all lots of products, including:
- LemonPrep
- PediaPrep
- Wave Prep
- Cardio Prep
- Collodions
- Collodion removers
These products are used in healthcare settings as skin prepping lotions, skin abrasives and cleaning agents. Healthcare providers use the products on surgical patients and those with electrodes on the skin. Also, the collodions remove skin impedances for electrodes, and the removers help remove the adhesive.
In October 2019, the U.S. Food and Drug Administration found Burkholderia cepacia on products during an inspection. In December 2019, Mavidon responded with a voluntary recall “out of an abundance of caution.” The company reports that it is unclear where the contamination originated, and therefore, all products made at their facility could be dangerous.
Mavidon distributes products to hospitals, clinics and other distributors. Consumers should stop using Mavidon products immediately. Products in the recall should be quarantined until Mavidon issues instructions for return or disposal.
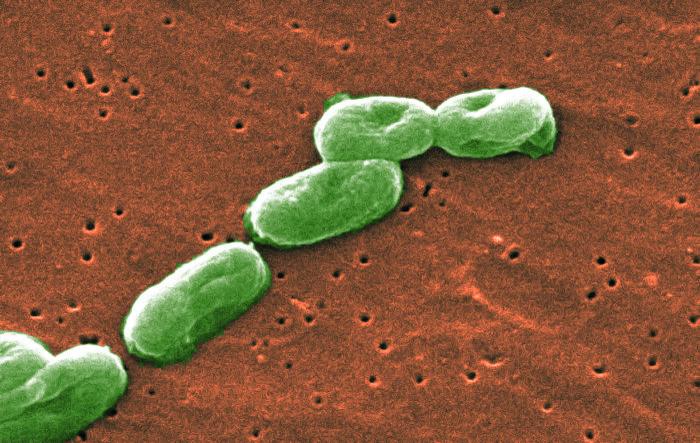
What is Burkholderia Cepacia?
Burkholderia cepacia is a dangerous pathogenic microorganism. The Centers for Disease Control and Prevention (CDC) classifies Burkholderia cepacia as a group of bacteria that exists in water and soil. It is often transmitted in healthcare settings due to contamination of medical devices or medications. In recent years, there have been several “outbreaks” of healthcare-associated infections due to contamination.
Burkholderia cepacia can also transmit through contact with surfaces, person-to-person contact and environmental exposure. It is multi-drug resistant and can result in serious infections. Treating infections is done on a case-by-case basis, depending on the patient’s overall health and other factors.
Certain populations are more at risk to the dangers of Burkholderia cepacia, including:
- Pregnant women
- Neonates
- Elderly
- Cancer patients
- People with weak immune systems
- People with chronic lung diseases (cystic fibrosis)
These individuals are more at risk for serious infections, but even healthy individuals can become seriously ill due to Burkholderia cepacia. So far, Mavidon has received one report of an adverse event in a neonate. Details of the report are unknown.
What to Do About Recalled Mavidon Medical Devices
Mavidon and the FDA urge healthcare providers and facilities to check their medical devices to see if they are subject to this recall. Furthermore, no one should use the products in the recall. Healthcare providers or consumers who experience an adverse event after using a Mavidon product should get medical treatment right away.
Because it is drug-resistant, if you receive a diagnosis of Burkholderia cepacia infection, it is important to get treatment immediately. Reports of infection should also be sent to the FDA. The FDA is accepting adverse event reports through their MedWatch Adverse Event Reporting Program. Consumers can also contact Mavidon directly at 1-800-654-0385.
Have Questions about Recalled Medical Devices?
If you have an injury or illness due to a medical device, you may be unsure of what to do next. At Drug and Device Watch, we can help. If you have questions about medical devices, recalls or your rights as a patient or consumer, contact us to learn more. Our legal professionals work hard to help you protect your rights and can certainly answer your questions.
To get answers to your questions, call us at 1-888-458-6825, or complete our online form for a free and confidential case evaluation.
Sources:
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/mavidon-issues-voluntary-worldwide-recall-all-manufactured-products-due-burkholderia-cepacia
- https://www.cdc.gov/hai/organisms/bcepacia.html
- https://www.fda.gov/node/360543
- https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/mavidon-issues-voluntary-worldwide-recall-lemonprepr-tubes-and-single-use-cups
- https://www.medmalfirm.com/news-and-updates/healthcare-infections-sepsis-in-hospitals/
